Management guidelines for endocrine irAEs
Common endocrine irAE symptoms
International guideline (ASCO, ESMO and NCCN) recommendations for endocrine irAEs1-3^
^ For detailed guidelines, please refer to original publication
| Hypothyroidism | Assessment | Management |
|---|---|---|
| Subclinical hypothyroidism |
TSH between 4 to <10 Normal FT4 Patient asymptomatic |
|
|
Elevated TSH (>10) Normal FT4 |
|
|
| Overt hypothyroidism |
Elevated TSH (>10) Low FT4 |
|
| Central hypothyroidism |
Normal or low TSH Low FT4 |
|
| Thyrotoxicosis | Assessment | Management |
| Lab-based diagnosis | Low or suppressed TSH with high FT4/total T3 |
|
FT4, free thyroxine; NCCN, National Comprehensive Cancer Network; T3, triiodothyronine; TFT, thyroid function test; TSH, thyroid-stimulating hormone.
| Assessment | Management | |
|---|---|---|
| Hypothyroidism |
Low FT4 with elevated TSH or TSH >10 with normal FT4 |
|
|
Thyrotoxicosis |
DDx thyroiditis, Graves diseases |
|
DDx, differential diagnosis; FT4, free thyroxine; ICI, immune checkpoint inhibitor; TSH, thyroid-stimulating hormone.
| Hypothyroidism | Assessment | Management |
|---|---|---|
| Grade 1 |
TSH>4.5 and <10 mIU/L and asymptomatic |
|
|
Grade 2 |
Moderate symptoms, able to perform ADL. TSH persistently >10 mIU/L |
|
|
Grade 3–4 |
Severe symptoms, medically significant or life-threatening consequences, unable to perform ADL |
|
| Thyrotoxicosis | Assessment | Management |
| Grade 1 |
Asymptomatic or mild symptoms |
|
| Grade 2 |
Moderate symptoms, able to perform ADL |
|
| Grade 3–4 | Severe symptoms, medically significant or life-threatening consequences, unable to perform ADL |
|
ADL, activities of daily living; ASCO, American Society of Clinical Oncology; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
| Assessment | Diagnosis | Management |
|---|---|---|
|
New-onset fasting glucose >200 mg/dL Random blood glucose >250 mg/dL History of T2DM with fasting/random glucose >250 mg/dL |
Consider new-onset ICI-T1DM Measure C-peptide with repeat serum glucose Evaluate for DKA via blood pH, metabolic panel, and urine/serum ketones Consider measurement of autoantibodies |
C-peptide low + DKA present Hold immunotherapy until DKA resolves Inpatient care Urgent endocrine consultation Manage DKA as per institutional guidelines Initiate insulin Close glucose monitoring |
|
C-peptide low + DKA not present Continue immunotherapy Urgent endocrine consultation Consider inpatient care Initiate insulin and close glucose monitoring |
||
|
C-peptide appropriate for serum glucose Continue immunotherapy Continue monitoring of serum glucose and consider HgbA1c Consider insulin resistance (T2DM) or steroid-related hyperglycaemia Consider medical therapy, diet and lifestyle interventions |
DKA, diabetic ketoacidosis; DM, diabetes mellitus; HgbA1c, hemoglobin A1c; ICI, immune checkpoint inhibitor; NCCN, National Comprehensive Cancer Network; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
| Assessment | Management | |
|---|---|---|
| Grade 1 |
Asymptomatic or mild symptoms T2DM with fasting glucose value >ULN to
160mg/dL
No evidence of CIADM such as ketoacidosis or laboratory evidence of pancreatic autoimmunity |
|
| Grade 2 |
Moderate symptoms, able to perform ADL; T2DM with fasting glucose value
>160–250 mg/dL
No ketoacidosis or metabolic derangements but other evidence of CIADM at any glucose level |
|
| Grade 3–4 |
Severe symptoms
Medically significant or life-threatening consequences Unable to perform ADL Grade 3: >250–500 mg/dL Grade 4: >500 mg/dL |
|
ADL, activities of daily living; ASCO, American Society of Clinical Oncology; CIADM, checkpoint inhibitor-associated diabetes mellitus; DKA, diabetic ketoacidosis; ED, emergency department; T2DM, type 2 diabetes mellitus; ULN, upper limit of normal.
| Assessment | Management |
|---|---|
|
Evaluate for symptoms
Measure cortisol and ACTH, TSH, FT4, and sex hormones as appropriate Cosyntropin stimulation testing is not recommended in acute settings Brain MRI ± contrast with pituitary/sellar cuts |
|
ACTH, adrenocorticotropic hormone; FT4, free thyroxine; IV, intravenous; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network; TSH, thyroid-stimulating hormone.
| Grade | Assessment | Management |
|---|---|---|
| Grade 1 | Vague symptoms, no headache or asymptomatic |
|
| Grade 2 | Moderate symptoms such as headache but no visual disturbance |
|
| Grade 1–2 | Fatigue or mood alteration but haemodynamically stable, no electrolyte distribution |
|
| Grade 3–4 | Severe mass effect symptoms such as severe headache, any visual disturbance, severe hypoadrenalism |
|
ESMO, European Society for Medical Oncology; IV, intravenous; MRI, magnetic resonance imaging; TFT, thyroid function test.
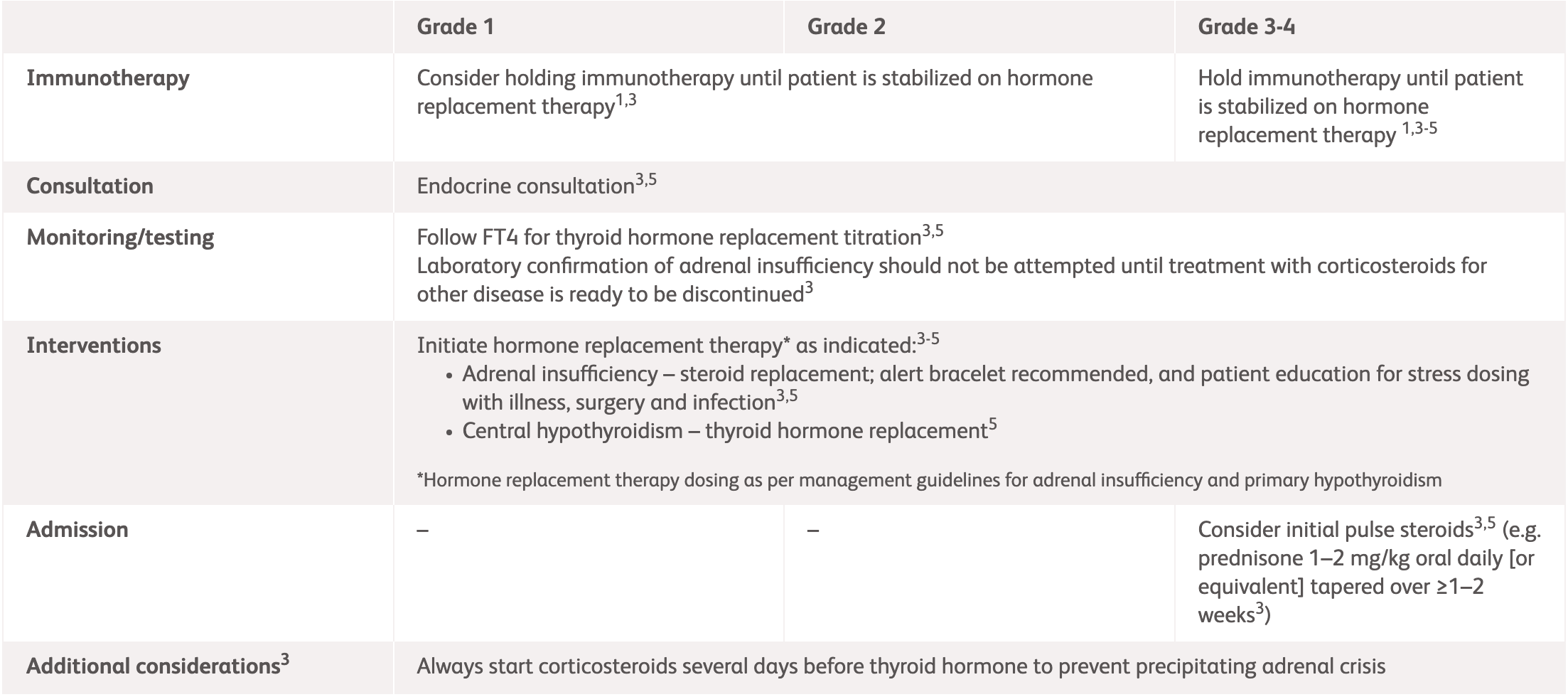
| Grade | Assessment | Management |
|---|---|---|
| Grade 1 | Asymptomatic or mild symptoms |
|
| Grade 2 | Moderate symptoms, able to perform ADL |
|
| Grade 3–4 | Severe symptoms, medically significant or life-threatening consequences, unable to perform ADL |
|
2L, 2 litres; ADL, activities of daily living; ASCO, American Society of Clinical Oncology; DI, diabetes insipidus; IV, intravenous; MRI, magnetic resonance imaging, Q6–8 , every 6 to 8 hours.
| Grade | Assessment | Management |
|---|---|---|
| Grade 1 | Asymptomatic or mild symptoms |
|
| Grade 2 | Moderate symptoms, able to perform ADL |
|
| Grade 3–4 | Severe symptoms, medically significant or life-threatening consequences, unable to perform ADL |
|
*NCCN and ESMO do not provide specific guidelines for primary adrenal
insufficiency.
2L,
2 litres;
ADL,
activities of daily living;
AI
, adrenal insufficiency;
ASCO,
American Society of Clinical Oncology;
Q6–8,
every 6 to 8 hours.
References:
- Schneider BJ, et al. J Clin Oncol 2021;39:4073–4126. Available at: https://ascopubs.org/doi/full/10.1200/JCO.21.01440. Accessed April 2025.
- Haanen J, et al. Ann Oncol 2022;33:1217–1238. Available at: https://www.annalsofoncology.org/article/S0923-7534(22)04187-4/fulltext. Accessed April 2025.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Management of immunotherapy-Related Toxicities. Version 1.2025. Available at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed April 2025.
- OPDIVO® (nivolumab) Product Information, BMS Hong Kong.
- YERVOY® (ipilumab) Product Information, BMS Hong Kong.