Management guidelines for neurologic irAEs
Common neurologic irAE symptoms
International guideline (ASCO, ESMO and NCCN) recommendations for neurologic irAEs1–3 ^
^ For detailed guidelines, please refer to original publication
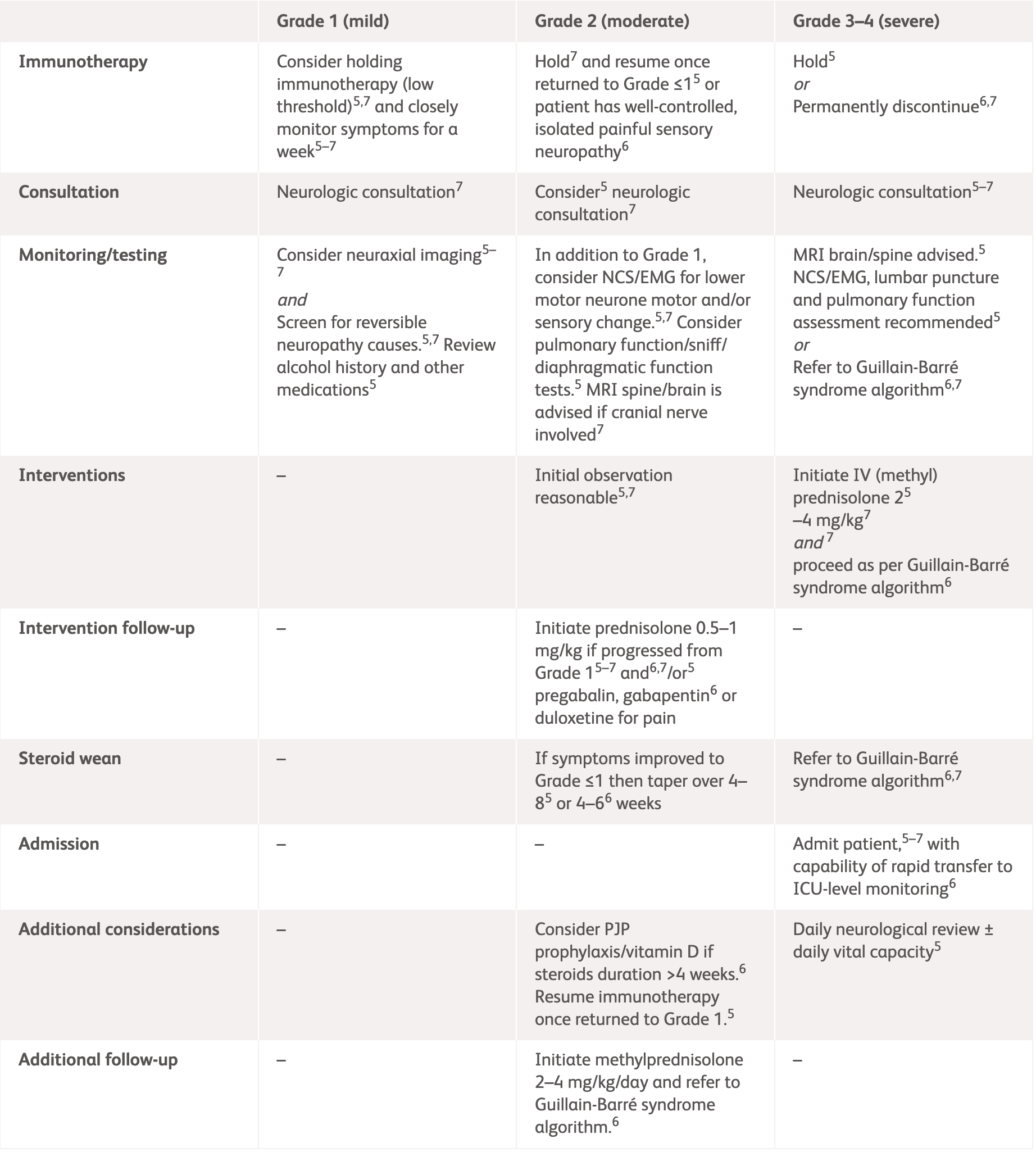
| Grading | NCCN2 | ASCO3 |
Grade 1
Mild symptoms, no interference with function |
|
|
|---|---|---|
|
Grade 2
Some interference with ADLs, symptoms concerning to the patient such as pain but no weakness or gait limitation |
|
|
|
Grade 3–4
Limiting self-care and aids warranted, weakness limiting walking or respiratory problems |
|
|
*ESMO does not provide guidelines for specific neurologic adverse events. Please see the specific guidelines to view their general recommendations.
ADLs, activities of daily living; ANCA, antineutrophil cytoplasmic antibodies; ASCO, American Society of Clinical Oncology; EMG, electromyography; ESMO, European Society for Medical Oncology; GBS, Guillain-Barré syndrome; HgbA1c, haemoglobin A1c; HIV, human immunodeficiency virus; IV, intravenous; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network; NCS, nerve conduction studies; SPEP, serum protein electrophoresis.
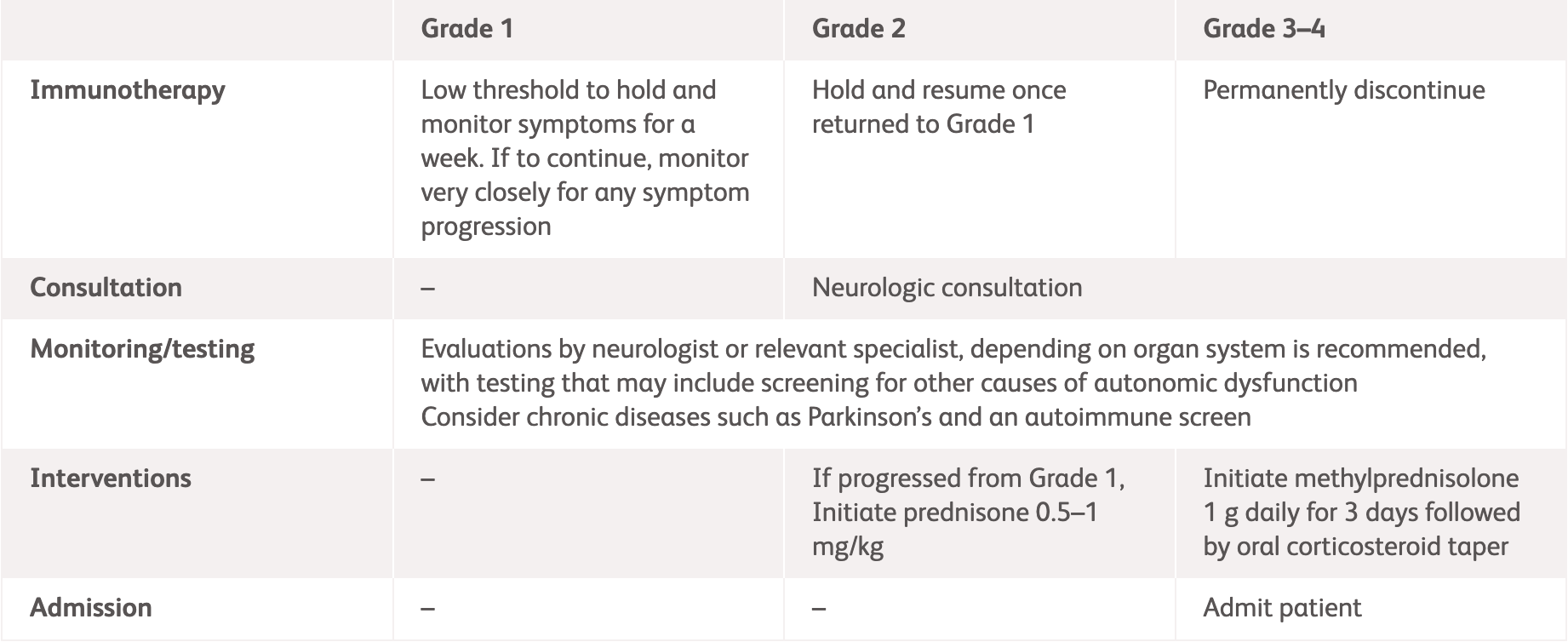
| ASCO3 | ||
|---|---|---|
| Grading | Management | |
| Grade 1 |
|
|
| Grade 2 |
|
|
| Grade 3–4 |
|
|
*ESMO does not provide guidelines for specific neurologic adverse events. Please see the specific guidelines to view their general recommendations.
ADLs, activities of daily living; ASCO, American Society of Clinical Oncology; ESMO, European Society for Medical Oncology.
| Grading | NCCN2 | ASCO3 |
|---|---|---|
|
Grade 1
Mild symptoms, no interference with function and symptoms not concerning to patient |
|
|
|
Grade 2
Moderate symptoms, some interference with ADLs, symptoms concerning to patient |
||
|
Grade 3–4
Severe symptoms, limiting self-care and aids warranted |
|
*ESMO does not provide guidelines for specific neurologic adverse events. Please see the specific guidelines to view their general recommendations.
ACTH, adrenocorticotropic hormone; ADLs, activities of daily living; ASCO, American Society of Clinical Oncology; CSF, cerebrospinal fluid; ESMO, European Society for Medical Oncology; IV, intravenous; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network.
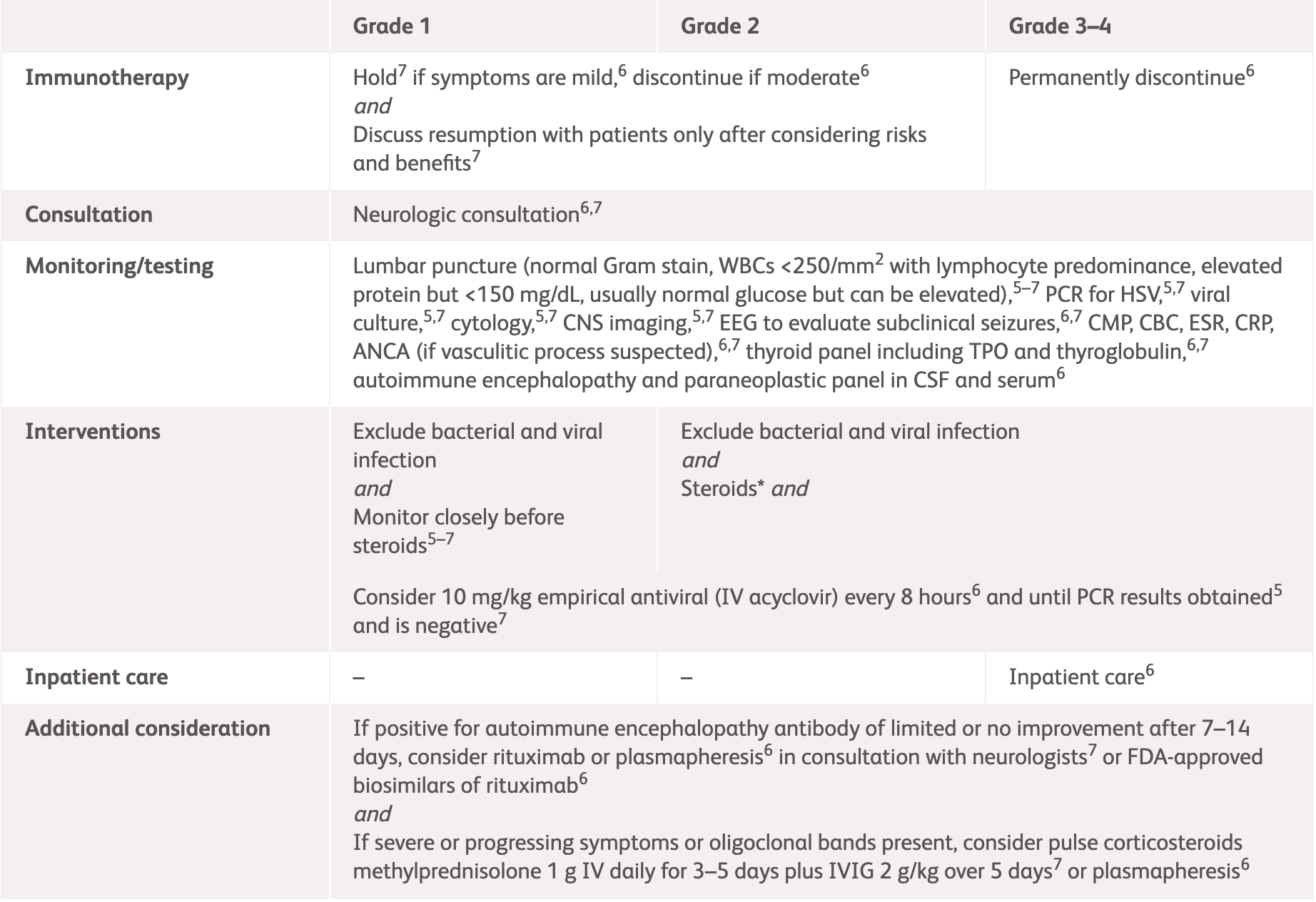
| Grading | NCCN2 | ASCO3 |
|---|---|---|
|
Grade 1
Mild symptoms, no interference with function and symptoms not concerning to patient3 |
|
|
|
Grade 2
Moderate symptoms, some interference with ADLs, symptoms concerning to patient3 |
||
|
Grade 3–4
Severe symptoms, limiting self-care and aids warranted3 |
*ESMO does not provide guidelines for specific neurologic adverse events. Please see the specific guidelines to view their general recommendations.
ADLs, activities of daily living; ANCA, anti-neutrophil cytoplasmic antibodies; ASCO, American Society of Clinical Oncology; CRP, C-reactive protein; CSF, cerebrospinal fluid; EEG, electroencephalogram; ESMO, European Society for Medical Oncology; ESR, erythrocyte sedimentation rate; IV, intravenous; IVIG, intravenous immunoglobulin; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network.
| Grading | Management | Assessment |
|---|---|---|
|
Grade 1
Asymptomatic or mild symptoms; clinical or diagnostic observations only |
|
|
|
Grade 2
Moderate symptoms, minimal, limiting age-appropriate instrument ADL |
|
|
|
Grade 3
Severe or medically significant symptoms, limiting self-care ADL |
|
|
|
Grade 4
Life threatening consequences |
|
ADL, activities of daily living; CSF, cerebrospinal fluid; EEG, electroencephalogram. ICU, intensive care unit; IVIG, intravenous immunoglobulin; MRI, magnetic resonance imaging; PCR, polymerase chain reaction.
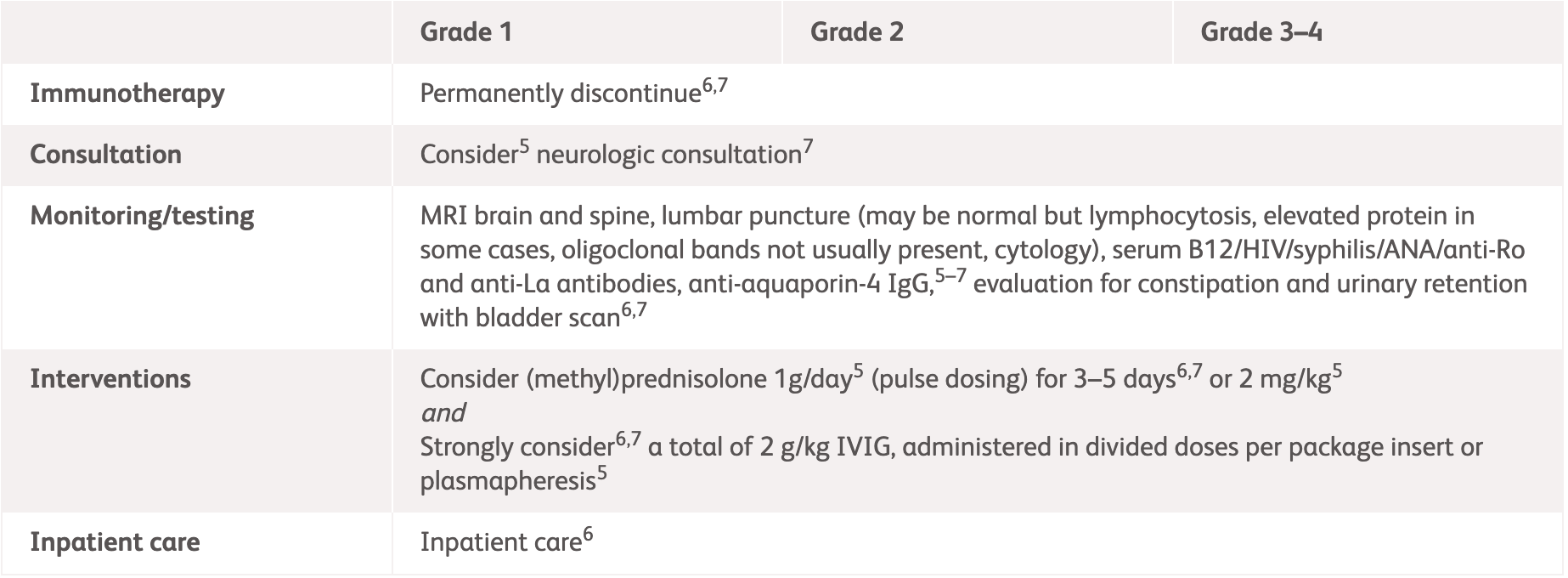
| Diagnosis | Assessment and Management |
|---|---|
| Acute or subacute weakness or sensory changes bilaterally, often with bowel/bladder changes and spinal level to pinprick, hyperreflexia, positive Babinski |
|
*No grading for transverse myelitis provided in NCCN guidelines.
IV, intravenous; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network.
| NCCN2 | ASCO3 | |
|---|---|---|
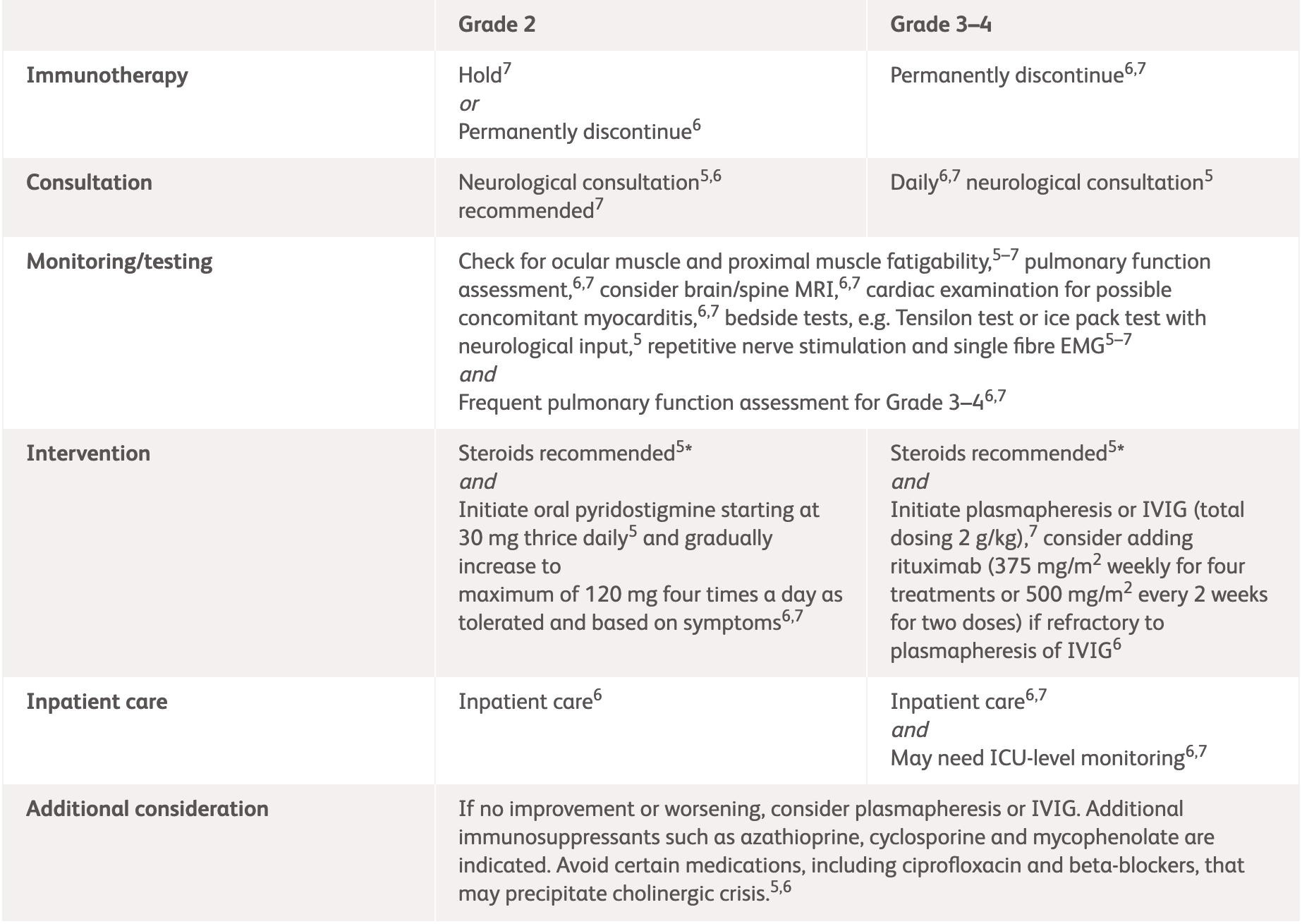
| Assessment | Neurology consultation Test for AChR, MuSK, and antistriational antibodies Pulmonary function assessment with NIF and VC Evaluate for concomitant myocarditis and myositis EMG/nerve conduction study Consider MRI Consider paraneoplastic workup Assess ESR and CRP |
|
|
Grade 2
Some symptoms interfering with ADLs MGFA severity class I and II |
|
|
|
Strongly consider inpatient care |
||
|
Grade 3–4
Limiting self-care and aids warranted, weakness limiting walking, any dysphagia, facial weakness, respiratory muscle weakness, or rapidly progressive symptoms MGFA severity class III–IV |
Permanently discontinue immunotherapy Admit patient (consider ICU-level monitoring) Initiate IVIG 0.4 g/kg/day IV over 5 days or plasmapheresis for 5 days Consider rituximab if refractory to IVIG or plasmapheresis Frequent pulmonary function assessment Daily neurologic review Avoid medication that can worsen myasthenia |
|
|
|
|
*ESMO does not provide guidelines for specific neurologic adverse events. Please see the specific guidelines to view their general recommendations.
AChR, acetylcholine receptor; ADLs, activities of daily living; ASCO, American Society of Clinical Oncology; CRP, C-reactive protein; EMG, electromyography; ESMO, European Society for Medical Oncology; ESR, erythrocyte sedimentation rate; ICU, intensive care unit; IV, intravenous; IVIG, intravenous immunoglobulin; MGFA, Myasthenia Gravis Foundation of America; MuSK, muscle-specific kinase; NCCN, National Comprehensive Cancer Network; NIF, negative inspiratory force; VC, vital capacity.
| NCCN2 and ASCO3 | |
|---|---|
| Assessment |
|
|
Grade 2
Moderate symptoms, some interference with ADLs, symptoms concerning to patient |
|
|
Grade 3–4
Limiting self-care and aids warranted, weakness limiting walking, any dysphagia, facial weakness, respiratory muscle weakness, or rapidly progressive symptoms |
*ESMO does not provide guidelines for specific neurologic adverse events. Please see the specific guidelines to view their general recommendations.
†Corticosteroids are typically not recommended for idiopathic GBS, but a trial is reasonable for ICPi-related forms.
ADLs, activities of daily living; ASCO, American Society of Clinical Oncology; CSF, cerebrospinal fluid; EMG, electromyography; ESMO, European Society for Medical Oncology; GBS, Guillain-Barré syndrome; ICPi, immune checkpoint inhibitor; ICU, intensive care unit; IV, intravenous; IVIG, intravenous immunoglobulin; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network; NCS, nerve conduction studies; NIF, negative inspiratory force; VC, vital capacity.
References:
- Haanen J, et al. Ann Oncol 2022;33:1217–1238. Available at: https://www.annalsofoncology.org/article/S0923-7534(22)04187-4/fulltext. Accessed March 2025.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Management of immunotherapy-Related Toxicities. Version 1.2022. Available at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed 13 February 2025.
- Schneider BJ, et al. J Clin Oncol 2021;39:4073–4126. Available at: https://ascopubs.org/doi/full/10.1200/JCO.21.01440. Accessed March 2025.
- OPDIVO® (nivolumab) Product Information, BMS Hong Kong.
- YERVOY® (ipilumab) Product Information, BMS Hong Kong.