International guideline (NCCN, ESMO and ASCO) recommendations for skin irAEs1-3^
^For detailed guidelines, please refer to original publications
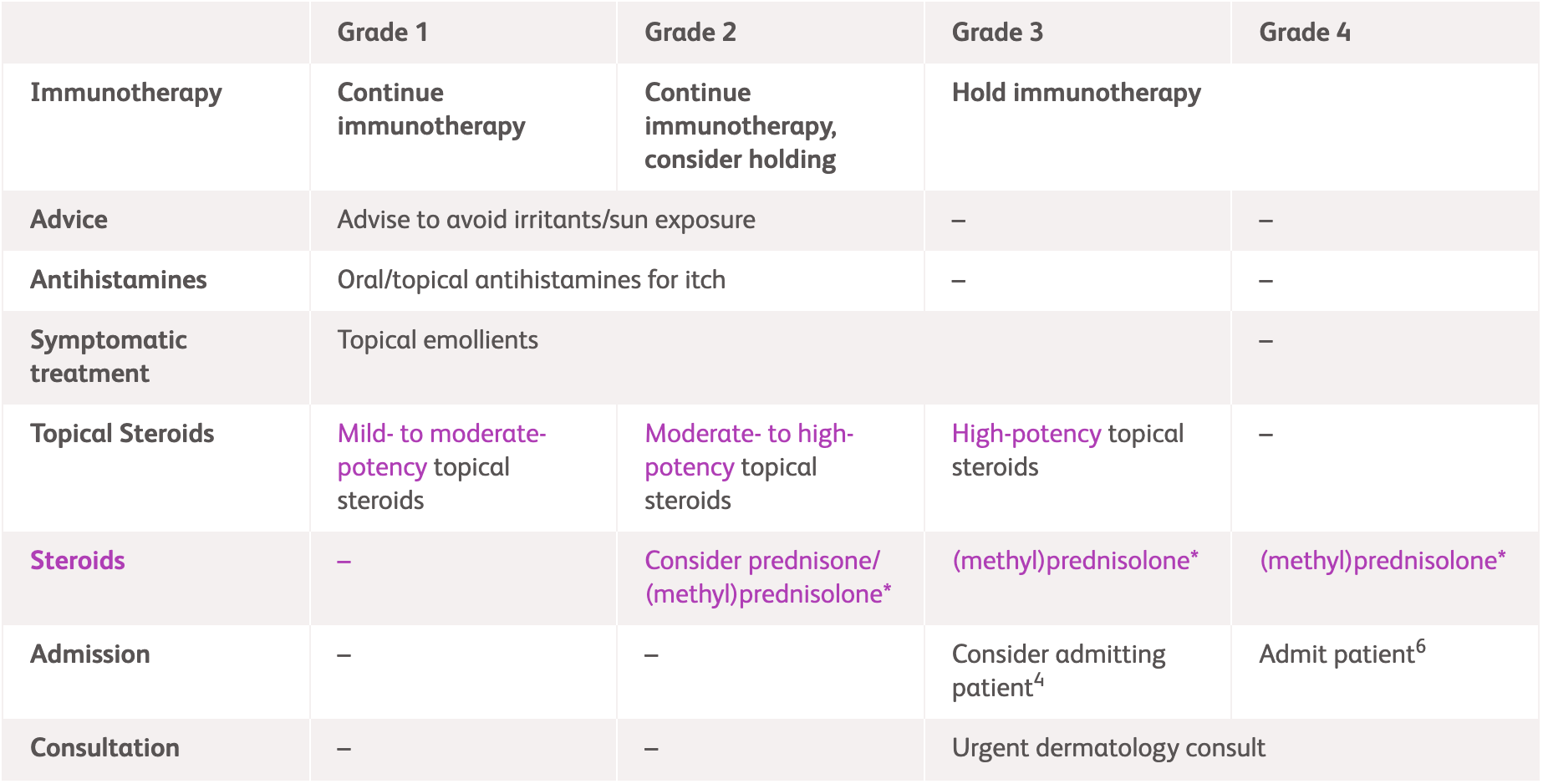
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Immunotherapy | Continue immunotherapy1-3 | Continue immunotherapy, consider holding1-3 | Hold immunotherapy1-3 | Immediately hold3 or permanently discontinue immunotherapy2 |
| Medication | Mild- to moderate-potency topical steroids1-3 | Moderate- to high-potency topical steroids1-3 | High-potency topical steroids1-3 | - |
| - | Consider prednisone/
(methyl)prednisolone1-3* |
Prednisone/(methyl)prednisolone1-3* | ||
| For Grade 1-2, oral antihistamines for itch1-3
For Grade 1-3, topical emollients1-3 |
||||
| Admission | - | - | Consider admitting patient1 | Admit patient2,3 |
| Consultation | - | - | Urgent dermatology consult1,3 | |
| Others | Advise to avoid irritants/sun exposure2,3 | - | - | |
*Consult steroid dosing information below for recommendations
Physical examination, history, total body skin exam including mucosa, skin biopsy, eosinophil count, peripheral blood smear and liver function tests.1-3 For grade 1, physical examination and exclude other causes.2 For grade 2, consider dermatology referral and skin biopsy and clinical photography.2 For grade 3, implement dermatology review and consider punch biopsy and clinical photography in addition to steps for earlier grades.2 For grade 4, conduct dermatology review, punch biopsy and clinical photography and all earlier steps2
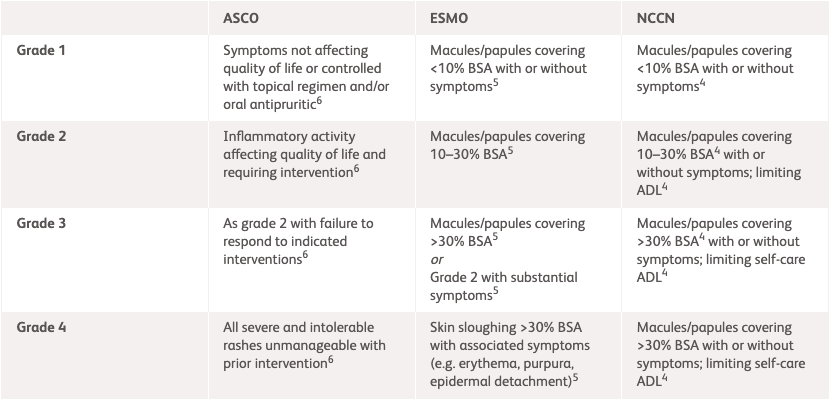
| NCCN1* | ESMO2 | ASCO3 | |
|---|---|---|---|
| Grade 1 | Macules/papules covering <10% BSA with or without symptoms | Lesions covering <10% BSA with or without symptoms | Rash covering <10% BSA, which may or may not be associated with symptoms of pruritus or tenderness |
| Grade 2 | Macules/papules covering 10-30% BSA with or without symptoms Limiting instrumental ADL |
Lesions covering 10-30% BSA with or without symptoms Limiting instrumental ADL Lesions covering >30% BSA with or without mild symptoms, without limiting self-care ADL |
Rash covering 10%-30% BSA with or without symptoms Limiting instrumental ADL Rash covering >30% BSA with or without mild symptoms |
| Grade 3 | Macules/papules covering >30% BSA with or without symptoms Limiting self-care ADL |
Lesions covering >30% BSA with moderate or severe symptoms Limiting self-care ADL |
Rash covering >30% BSA with moderate or severe symptoms Limiting self-care ADL |
| Grade 4 | - | Life-threatening consequences, urgent intervention needed | Severe consequences requiring hospitalisation or urgent intervention indicated or life-threatening consequences |
*NCCN grading for rashes as an immune checkpoint inhibitor-related toxicity only extends to Grade 3.
ADL, activities of daily living; ASCO, American Society of Clinical Oncology; BSA, body surface area; ESMO, European Society for Medical Oncology; NCCN, National Comprehensive Cancer Network.
| NCCN1* | ESMO2 | ASCO3 | |
|---|---|---|---|
| Grade 2 | Consider 0.5 mg/kg/day prednisone if unresponsive to topical treatments within 1-2 weeks | If refractory, initiate (methyl)prednisolone/prednisolone (or equivalent) 0.5-1 mg/kg tapering over >4 weeks Restart ICI therapy when grade 1 and prednisone <10 mg/day |
Consider prednisone (or equivalent) 0.5-1 mg/kg tapering over 4 weeks |
| Grade 3 | 0.5-1 mg/kg/day prednisone, up to 2 mg/kg/day if no improvement, tapering over 4-6 weeks once symptoms are Grade ≤1 | Initiate oral prednisone (or equivalent) 1 mg/kg/day tapering over at least 4 weeks | |
| Grade 4 | - | IV (methyl)prednisolone (or equivalent) 1-2 mg/kg tapering over >4 weeks once reaction is controlled. If refractory to CS, consider additional therapies such as infliximab or toxilizumab |
IV methylprednisolone (or equivalent) 1-2 mg/kg with slow tapering when the toxicity resolves |
*NCCN grading for rashes as an immune checkpoint inhibitor-related toxicity only extends to Grade 3
ASCO, American Society of Clinical Oncology; CS, corticosteroids; ESMO, European Society for Medical Oncology; IV, intravenous; NCCN, National Comprehensive Cancer Network.
|
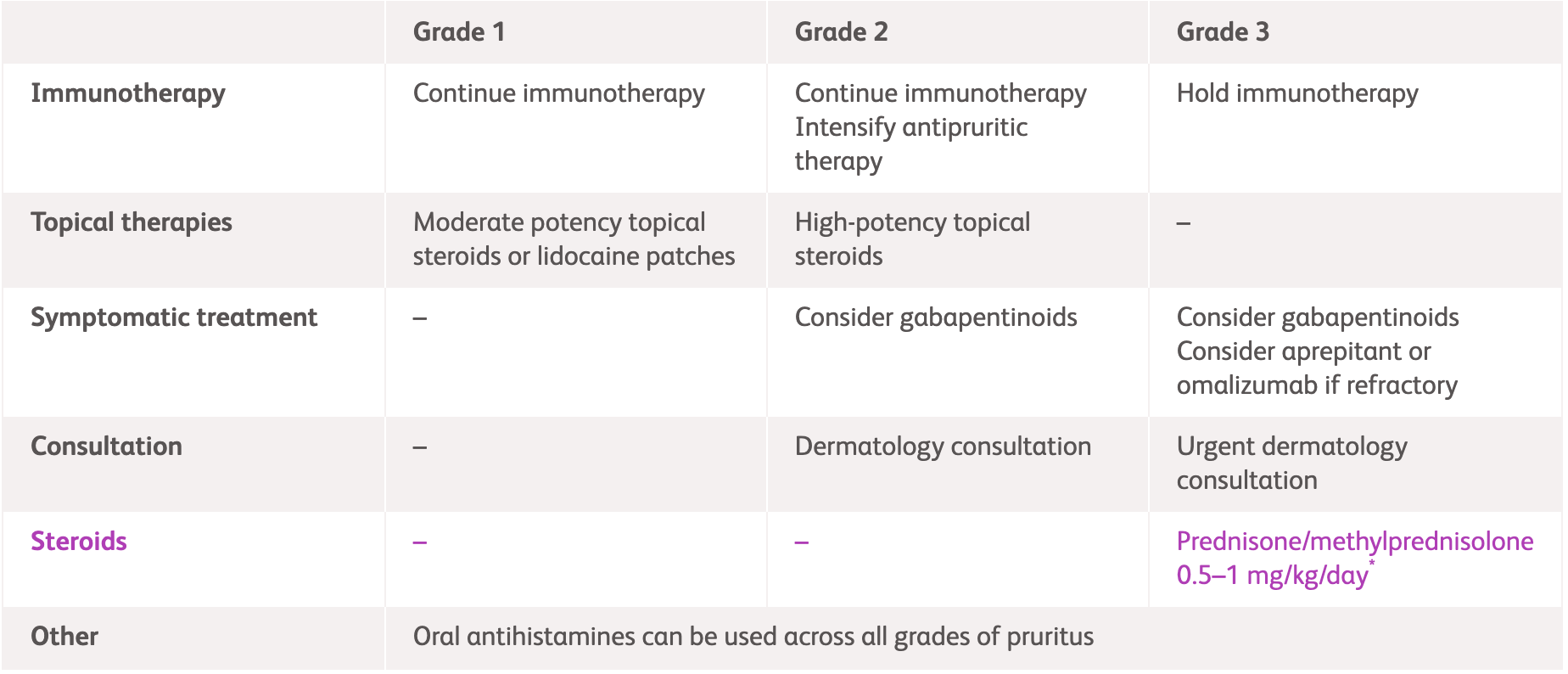
NCCN1 |
|||
|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |
| Immunotherapy | Continue immunotherapy | Hold immunotherapy | Hold immunotherapy |
| Intervention | Consider over-the-counter topical anaesthetic/anti-itch creams | Consider:
|
Prednisone/methylprednisolone 0.5-1 mg/kg/day* Consider gabapentin or pregabalin |
| Moderate-potency topical steroids to affected areas for localised pruritus | High-potency topical steroids | ||
| Oral antihistamines and whole body moisturisation with non-fragranced moisturiser can be used across all grades of pruritus | |||
| Consultation | - | Dermatology consultation | Urgent dermatology consultation |
*Continue until symptoms improve to Grade ≤1, then taper over 4-6 weeks.
NCCN, National Comprehensive Cancer Network; UVB, ultraviolet B
Assess pruritus through a total body skin exam including the mucosa, and assess for history of prior inflammatory dermatological diseases.
|
NCCN1 |
||
|---|---|---|
| Grade 1 | Grade 2 | Grade 3 |
| Mild or localized | Intense or widespread, intermittent; skin changes from scratching; limiting instrumental ADL | Intense or widespread; constant; limiting self-care ADL or sleep |
ADL, activities of daily living; NCCN, National Comprehensive Cancer Network.
|
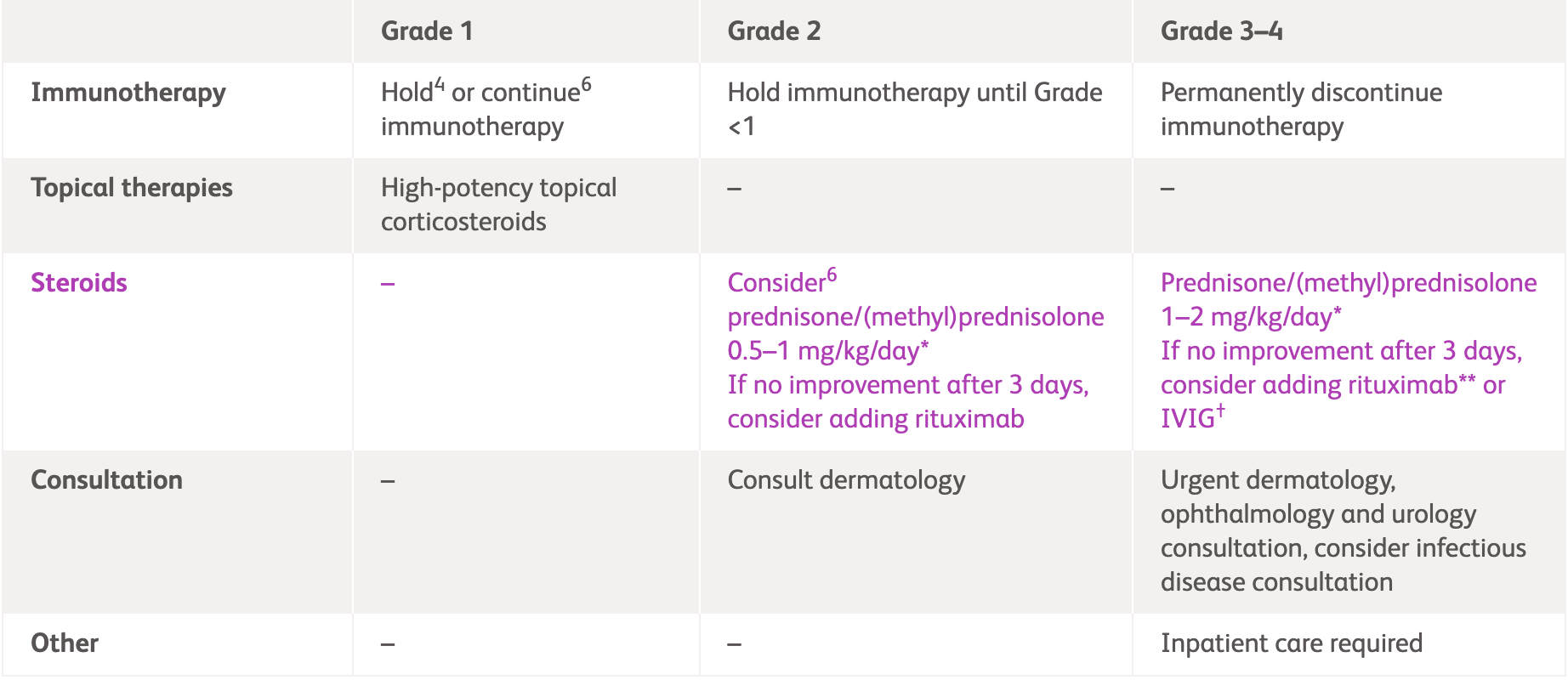
NCCN, ASCO1,3 |
|||
|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3-4 | |
| Immunotherapy | Consider holding1 or continue3 immunotherapy if asymptomatic | Hold immunotherapy until Grade <11,3 |
Grade 3: Hold3 or discontinue1 immunotherapy Grade 4: Discontinue immunotherapy1,3 |
| Medication | High-potency topical corticosteroids1 | Class 1 high-potency topical steroid(clobetasol, betamethasone or equivalent)3 |
IV methylprednisolone (or equivalent) 1-2 mg/kg1,3 and when appropriate convert to oral steroids, tapering over at least 4 weeks3* If bullous pemphigoid is diagnosed, consider steroid-sparing options (e.g, IVIG and rituximab)3 Consider IVIG (1 g/kg/day x 2 days with monthly cycle until clear) as an adjunct to rituximab or dupilumab1** |
|
- |
Prednisone/IV methylprednisolone 0.5-1 mg/kg/day1,3* General local wound care, including plain petrolatum ointment and bandages3 | ||
| Consultation | - | Consult dermatology3 | Urgent dermatology consultation1,3 |
| Other | - | - | Inpatient care required1,3 |
*Until symptoms resolve to Grade ≤1, tapering over 4-6 weeks; **Rituximab at 1,000 mg every 2 weeks for two doses in combination with tapering of glucocorticoids, followed by 500 mg maintenance rituximab at 12 and 18 months.
IV, intravenous immunoglobulin; IVIG, intravenous immunoglobulin.
Physical examination, ruling out other aetiologies. Dermatology consultation, skin biopsy, serology.
|
NCCN & ASCO1,3 |
|||
|---|---|---|---|
| Grade 1 | Grade 2* | Grade 3 | Grade 4 |
| Asymptomatic, blisters covering <10% BSA1,3 No associated erythema3 |
Blistering that affects quality of life and requires intervention based on diagnosis not meeting criteria for Grade >2.3 Blisters covering 10-30% BSA1,3 | Skin sloughing and blistering covering >30% BSA with associated pain and limiting self-care ADL1,3 | Blisters covering >30% BSA with associated fluid or electrolyte abnormalities1,3 ICU care or burn unit indicated1 |
*When symptomatic bullae or erosions, which are deroofed vesicles or bullae, are noted on the skin or mucosal surfaces, the cutaneous irAE is considered at least grade 2.3
ADL, activities of daily living; ASCO, American Society of Clinical Oncology; BSA, body surface area; ICU, intensive care unit; irAE, immune-related adverse events; NCCN, National Comprehensive Cancer Network.
NCCN guidelines1
| All grades | |
|---|---|
| Immunotherapy | Permanently discontinue immunotherapy |
| Medication | Prednisone/(methyl)prednisolone 1-2 mg/kg/day
Consider IVIG* |
| Consultation | Urgent dermatology, ophthalmology and urology consultation |
| Other | Inpatient care required |
*IVIG 1 g/kg/day in divided doses per product information for 3-4 days. Other immunosuppressive therapies (i.e., etanercept, cyclosporine) can be considered. After a patient has widespread skin separation (blisters or erosions), the risk of infection should be weighed against the potential benefits of immunosuppression.
ASCO guidelines3
In cases of suspected SJS or any mucous membrane involvement (not including isolated stomatitis), discontinue immunotherapy and consult dermatology. Monitor closely for improvement regardless of grade.
| Grade 1-2* | Grade 3 | Grade 4 | |
|---|---|---|---|
| Immunotherapy | - | Hold immunotherapy | Permanently discontinue immunotherapy |
| Topical therapies | - | Topical emollients and other petrolatum emollients, oral antihistamines, and high-strength topical corticosteroids. Dimethicone may be offered as an alternative to petrolatum. |
- |
| Medication | - | IV (methyl)prednisolone 0.5-1 mg/kg
Convert to oral corticosteroids on response and taper for ≥4 weeks |
IV (methyl)prednisolone 1-2 mg/kg, tapering when toxicity resolves
For corticosteroid-unresponsive cases, consider IVIG or cyclosporine |
| Admission | - | Admit to burn unit and/or consult wound services with attention to supportive care including fluid and electrolyte balance, minimizing insensible water losses, and preventing infection. | Admit to burn unit/ICU, consult dermatology and wound care services |
| Consultation | - | Consult widely, as appropriate, for management of mucosal surfaces and preventing sequelae For patients with DRESS, consider pain/palliative consultation and/or admission |
|
*For the SCAR adverse reactions, there are no Grade 1 or 2 categories. If limited BSA is involved with bullae or erosions, there should remain high concern that this reaction will progress to grade 3 or 4.3
AE, adverse event; ASCO, American Society of Clinical Oncology; BSA, body surface area; DRESS, drug reaction with eosinophilia and system symptoms; ICU, intensive care unit; IV, intravenous; IVIG, intravenous immunoglobulin; NCCN, National Comprehensive Cancer Network; SCAR, severe cutaneous adverse reaction.
An urgent dermatology consultation is required at the first signs or symptoms of SJS or TEN. Patients should undergo total body skin and mucous membrane examinations, ruling out other aetiologies, serology/biologic check-ups, and skin biopsy.
|
ASCO3 |
|||
|---|---|---|---|
| Grade 1-2* | Grade 3 | Grade 4 | |
| - | Skin sloughing covering <10% BSA with mucosal involvement-associated signs (e.g. erythema, purpura, epidermal detachment, mucous membrane detachment) | Skin erythema and blistering/sloughing covering ≥10% BSA with associated signs (e.g. erythema, purpura, epidermal detachment, mucous membrane detachment) and/or systemic symptoms and concerning associated bloodwork abnormalities (e.g. liver function test elevations in the setting of DRESS/DIHS) | |
*For the SCAR adverse reactions, there are no Grade 1 or 2 categories. If limited BSA is involved with bullae or erosions, there should remain high concern that this reaction will progress to grade 3 or 4.3
ASCO, American Society of Clinical Oncology; BSA, body surface area; DIHS, drug-induced hypersensitivity syndrome; DRESS, drug reaction with eosinophilia and system symptoms; SCAR, severe cutaneous adverse reaction; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
References:
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Management of immunotherapy-Related Toxicities. Version 1.2025. Available: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed March 2025.
- Haanen J, et al. Ann Oncol 2022;33:1217–1238. Available at: https://www.annalsofoncology.org/article/S0923-7534(22)04187-4/fulltext. Accessed March 2025.
- Schneider BJ, et, al. J Clin Oncol 2021;39:4073–4126. Available at: https://ascopubs.org/doi/full/10.1200/JCO.21.01440. Accessed March 2025.
- OPDIVO® (nivolumab) Product Information, BMS Hong Kong.
- YERVOY® (ipilumab) Product Information, BMS Hong Kong.